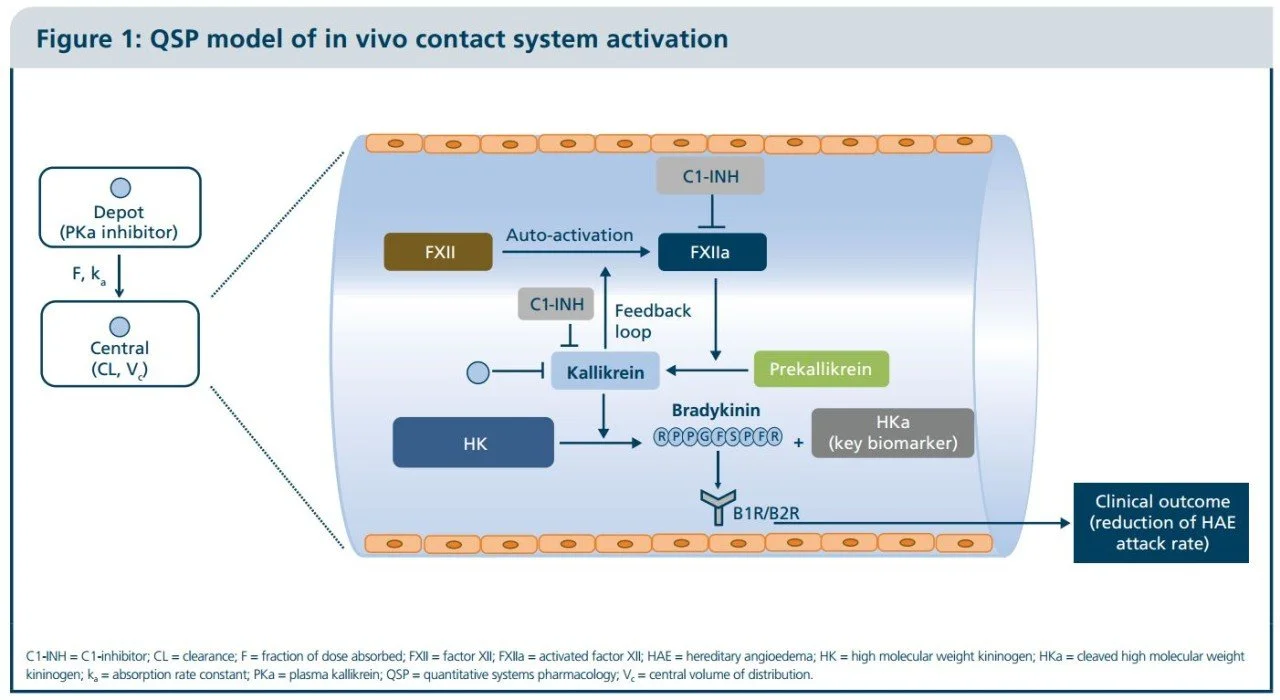
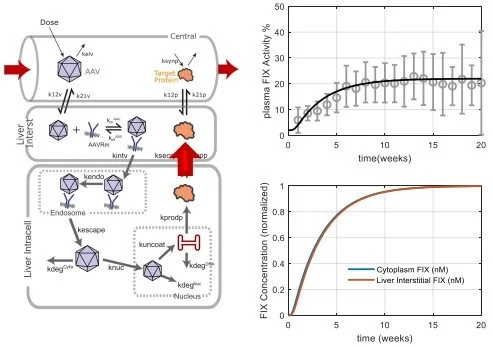
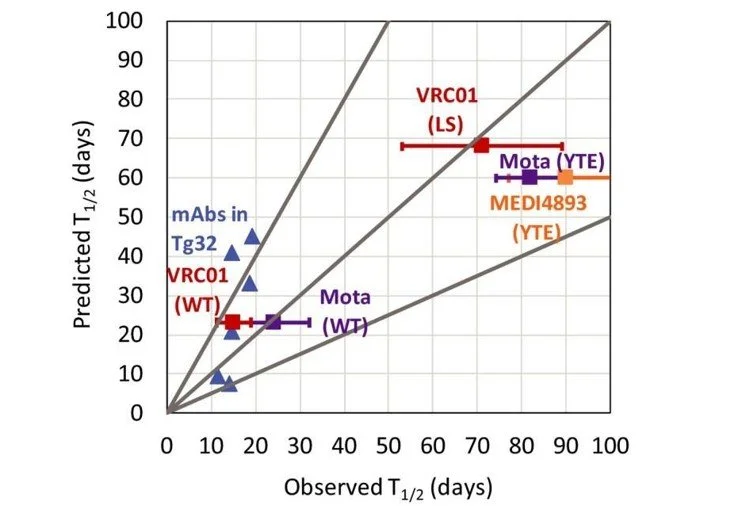
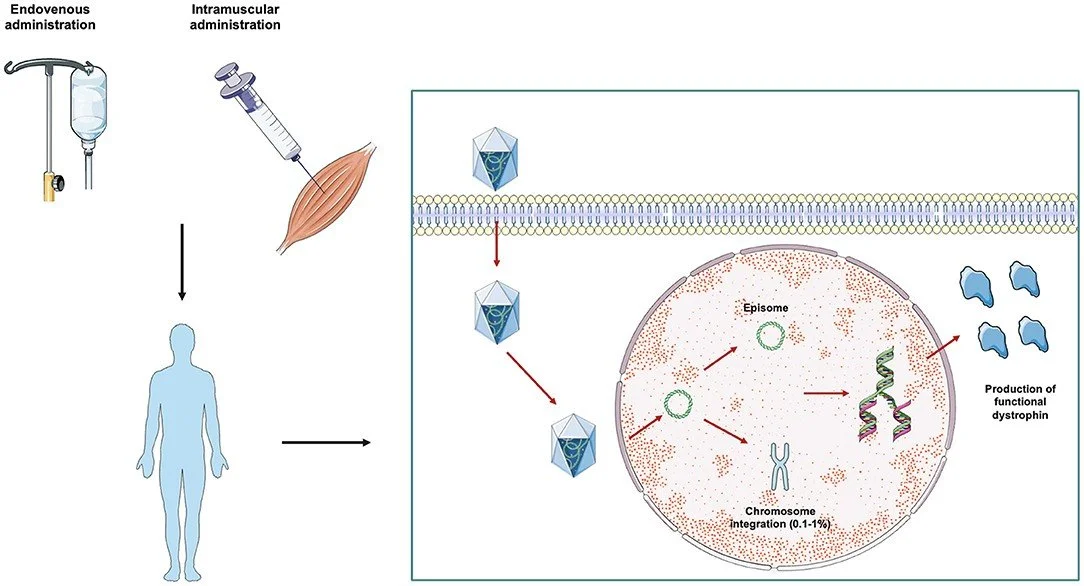
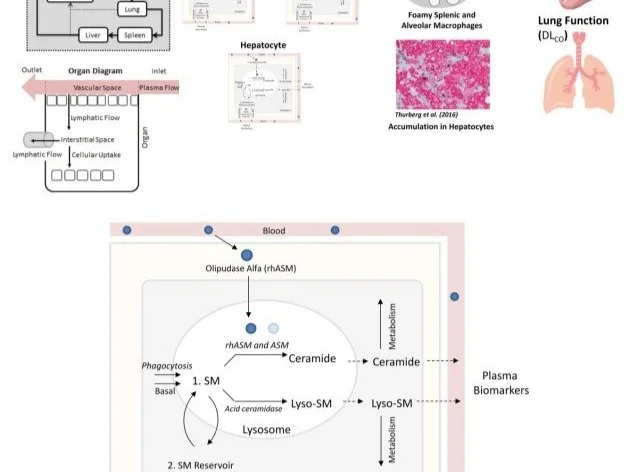
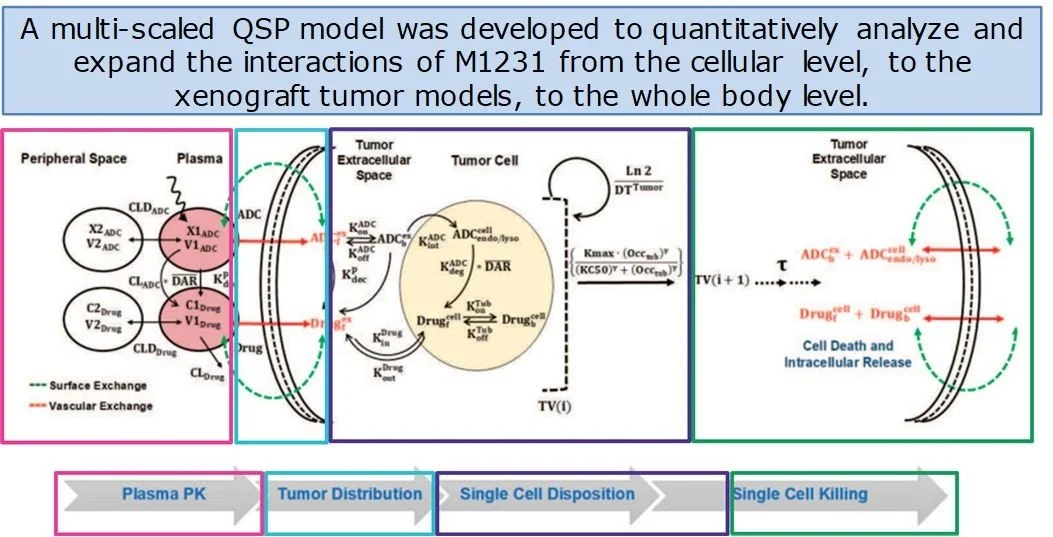
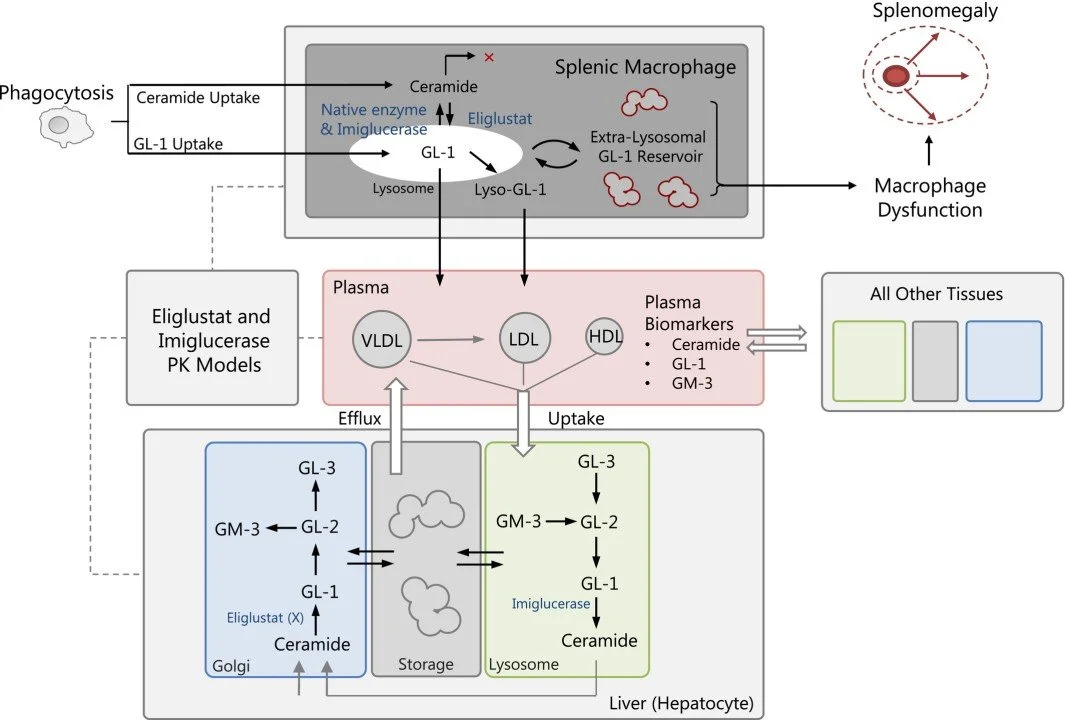
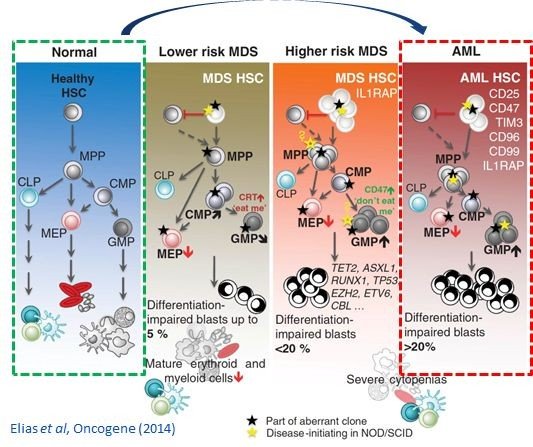
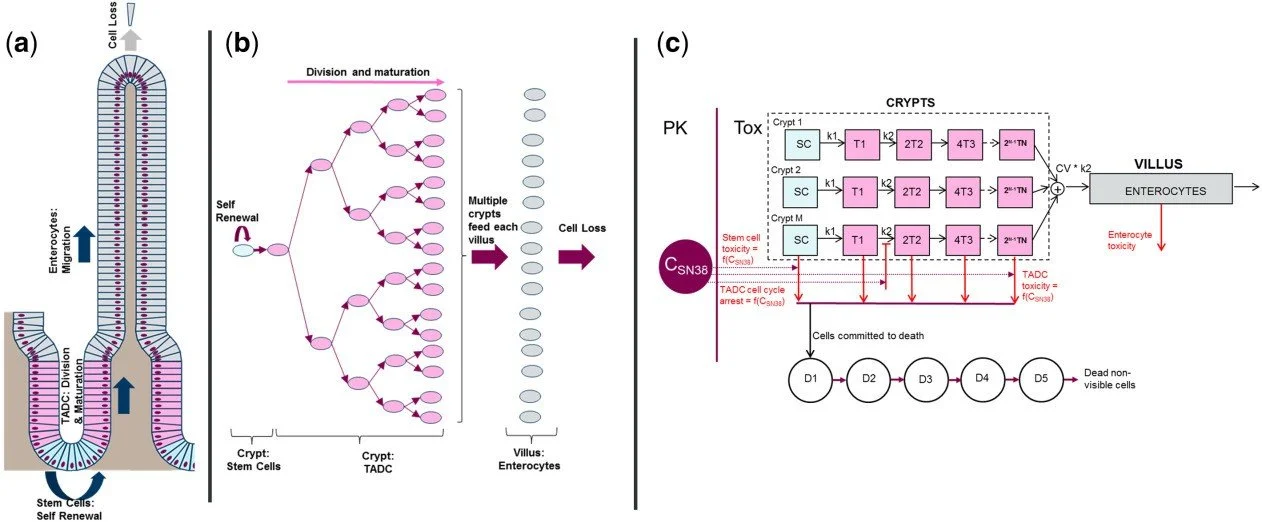
Over the past two decades, RES has developed an extensive range of quantitative systems pharmacology (QSP) models to assist in the development of new drugs
| ONCOLOGY | AUTOIMMUNITY & INFLAMMATION | RARE DISEASE | NEUROSCIENCE | INFECTIOUS DISEASE | TOXICITY | |
|---|---|---|---|---|---|---|
| INDICATIONS* | Non-small cell lung carcinoma (NSCLC) | Systemic lupus erythematosus (SLE) | Hereditary angioedema (HAE) | Alzheimer's disease | HCV | Gut toxicity |
| Small cell lung carcinoma (SCLC) | Lupus nephritis (LN) | Dilated Cardiomyopathy | Parkinson's disease | SARS-CoV-2/COVID-19 | Anemia | |
| Acute lymphoblastic leukemia (ALL) | IgA nephropathy (IgAN) | Niemann-Pick disease (LSD) | Narcolepsy | Neutropenia | ||
| Non-Hodgkin's lymphoma (NHL) | Nonalcoholic steatohepatitis (NASH) | Gaucher's disease (LSD) | Cataplexy | Thrombocytopenia | ||
| Acute myeloid leukemia (AML) | Asthma/ACOS | Fabry's disease (LSD) | Friedrich’s Ataxia | Leukopenia | ||
| Colorectal cancer (CRC) | Myasthenia gravis (MG) | Hunter's syndrome (LSD) | Huntington's disease (HD) | Crystal nephropathy | ||
| Breast cancer | Rheumatoid arthritis (RA) | Sickle cell anemia (SCD) | ||||
| Pancreatic cancer | Inflammatory bowel disease (IBD) | Duchenne Muscular Dystrophy | ||||
| DRUG MODALITIES* | Antibody-drug conjugates (ADC), including bispecifics | Cytokine-targeting mAbs & bispecific Abs | Gene therapy | Small molecule inhibitors | Small molecule inhibitors | Enterocyte model |
| T-cell engagers (BiTEs, bispecific Ab, etc.) | Chemokine-targeting mAbs | mAbs and engineered Abs | mAbs | mRNA vaccines | Hematopoiesis model | |
| Small molecule inhibitors (mEGFR, mALK, SHP2, etc.) | FcRn-targeting mAbs & Ab-fragments | Enzyme replacement therapy (ERT) | TfR-targeting drug conjugates (BBB) | Nephron model | ||
| Small molecule DNA repair inhibitors (ATRi, ATMi, etc.) | Tri specific large-molecule inhibitors | Substrate reduction therapy (SRT) | ||||
| Oncolytic viruses w/ immune modulating "cargo" | siRNA | TfR-targeting drug conjugates (RBC) | ||||
| Checkpoint inhibitor mAbs | Intracellular protein degraders | |||||
| PEG-conjugated cytokines | Extracellular protein degraders | |||||
| Lipid nanoparticles |
*Partial list
SCOPE OF OUR SERVICES
Scope:
Identify New Drug Targets. Treatment Strategies. Validate New Methodologies. Mechanism-of-action
Scope:
Drug Design Improvement, Next-Gen Drug Design, Experimental Design, Drug-Failure Analysis, PBPK Models
Scope:
Optimal Dose,
Dose Schedule,
Predict Effect Size,
Inclusion/Exclusion Critera, Biomarkers of Response
Scope:
Routes of Synthesis,
Crystallization,
Morphology Control,
Scale-Up,
Process Optimization
Drug Discovery &
Development
RES has deep expertise in the fields of drug discovery and development, helping our pharmaceutical partners bring new and innovative drugs to market.
Scope of Expertise:
Possible Causes
Mechanism of Action
Disease Progression
Treatment Possibilities
Novel Drug Target Identification
Drug Failure Analysis
Next-Gen Drug Design
Disease Area Expertise:
Cancer (Multiple Subtypes)
Autoimmunity
Parkinson's Disease
Alzheimer's Disease
Cardiovascular Disease
Chronic Pain
Osteoporosis & Bone
Remodeling
Lipoprotein Metabolism
Signal Transduction
Clinical Trials
Often the most important and costly step in the pharmaceutical developmental cycle, RES scientists can help extract insight from often-underutilized clinical trial data. These insights are then leveraged toward better design of subsequent clinical trials and in some cases, better next generation drug design.
Clinical Trial Expertise:
Drug Design/Revision
Drug Administration
Drug Distribution
Effectiveness
Safety
Optimal Dose
Trial Duration
Inclusion/Exclusion Criteria
Biomarkers of Response
Expected Effect Size
New PKPD Algorithm Development
PBPK Models of Drug Distribution